Background
It is well known that Bortezomib (B), a proteasome inhibitor used in the treatment of Multiple Myeloma (MM) causes peripheral neuropathy in an estimated 75% of patients and compromises their quality of life. There are reports of dizziness, muscle weakness and other unusual neurologic complications caused by B. Among patients that received B based induction, we observed peripheral neuropathy as expected. In addition, in a small subset we observed profound weight loss, dizziness, orthostatic hypotension (OH) and diarrhea. Some patients with weight loss required appetite stimulants and patients with OH/dizziness required medication for symptom control. The conglomeration of symptoms frequently impairs patients' performance status requiring interruption of therapy. B was given in combination with lenalidomide and dexamethasone (VRD) but we feel the neuropathic symptoms are more due to B since the combination of just lenalidomide and dexamethasone is not known to cause such complications. There are few publications on autonomic neuropathy (AN) caused by B but we believe it is under recognized and under diagnosed. Herein we report a series of patients managed at our center that presented with significant weight loss and dizziness suggestive of AN, prompting us to explore further and bring awareness to this problem.
Methods
From January 2018 to July 2023, patients with MM treated in our own practice and patients induced by our referring practices and sent to our center for transplant were evaluated. We identified patients who complained of significant weight loss and dizziness and reviewed medical records, looked at the characteristics they exhibited, and identified a pattern of symptoms and clinical course. Statistics are descriptive and this retrospective study was approved by our Institutional review board.
Results
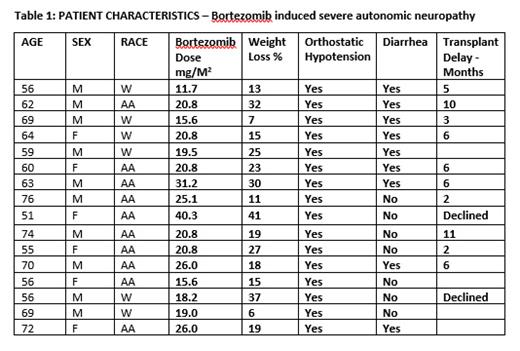
Sixteen patients were noted to have symptoms suggestive of AN. 13 of 16 received VRD and 3 received Daratumumab, B and dexamethasone-based induction. B was administered subcutaneously and given twice weekly. The median age was 62.5 years (range 51-76). Ten were African American and 6 White; 6 female, 4 had diabetes but no neuropathy, 10 had bone disease, 5 renal failure at presentation. The interval between initiation of therapy and AN symptoms was a median of 117 days (range 50-219) and the median B dose was 20.8 mg/M 2 (range 11.7-40.3 mg/M 2). All 16 patients had weight loss and was the most recognizable symptom. The median weight loss and percentage weight loss (compared to baseline weight) were 18.2 Kgs (range 6.7-39.14 Kgs) and 19% (range 6-41%) respectively. 10 of 16 patients had loss of appetite and 9 had diarrhea. Further treatment was held due to decline in performance status in 14 patients. Nine patients received appetite stimulants; 2 megestrol and 7 dronabinol. OH and an increase in heart rate in the standing position was seen in all 16 patients. 11 of the 16 patients had dizziness; 3 patients received fludrocortisone and one received midodrine. 14 of 16 patients also had peripheral neuropathy of whom 13 required medications for neuropathic pain and 14 required narcotic analgesics.
Conclusion
14 of 16 patients received a transplant and 2 declined. Transplant was delayed by 6 months (range 2-11) to enable recovery and the end-point was weight gain, resolution of OH and clinical improvement. Post-transplant 4 patients had persistent diarrhea and 1 had persistent dizziness. One patient developed severe mucositis, dysphagia, poor appetite and required hyperalimentation, nasogastric feeding and a feeding gastrostomy tube. 9 of 14 patients had persistent peripheral neuropathy and are using medications. Two of the 16 patients progressed and received salvage treatment one of the two is now deceased. With a median follow up of 26.35 (range 8.47-53.6) months, the median survival since diagnosis is 28.72 months.
Severe AN characterized by weight loss, dizziness, OH in association with loss of appetite, diarrhea and painful neuropathy is an under recognized complication of Bortezomib. This generally results in interruption of disease directed therapy and delays autologous transplantation. Transplantation without resolution of symptoms could possibly worsen the morbidity and delay post-transplant recovery. To the best of our knowledge, we are the first group to report a series of patients with this symptom complex and strongly feel it requires further study in a larger cohort.
Disclosures
Keruakous:BMS: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kota:Kite: Honoraria; Pfizer: Honoraria; Incyte: Research Funding; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal